As the spray season approaches, it is good to remember the profound impact water quality has on the performance of pesticides used by fruit growers. Purdue Pesticides Program recently published a guide, The Impact of Water Quality on Pesticide Performance PPP-86, available at the Education Store, 1-888-EXT-INFO or www.extension.purdue.edu/store/. I highly recommend this guide to all growers. Water quality can be broken down into a few categories. Most important to pesticide applicators are water hardness and pH.
Water hardness: Water hardness has a significant impact on several herbicides use by fruit growers. Hardness refers to the concentration of soluble salts, mainly calcium, magnesium, and iron in the water. Water is considered hard if it has 200 ppm or more of these positively charged (cation) minerals. Water is considered softened when the calcium and magnesium cations are replaced with sodium or potassium ions.
Hard water is common in many parts of Indiana. Fruit growers often apply a post-emergent herbicide beneath the tree or vine row in spring to control winter annuals and other weeds. Glyphosate (Roundup) is the most common post emergent systemic herbicide used in fruit crops. In order for glyphosate to be effective, it needs to be absorbed into the weed plant. In soft water weeds readily absorb glyphosate. However in hard water glyphosate will be ‘tied up’ and not absorbed as readily. When calcium and magnesium cations are present they react with the negatively charged glyphosate to form compounds that are not readily absorbed by plants. This results in poor uptake and poor weed control.
The solution to the hard water problem is to add ammonium sulfate to the spray water before mixing with glyphosate. Ammonium sulfate ions combine with the calcium and magnesium ions forming conjugate salts. Additionally, some of the glyphosate reacts with ammonium to form a compound that some weeds preferentially absorb. Sprayable ammonium sulfate (AMS) is available in granular and liquid formulations. Follow the label recommendations on the amount of ammonium sulfate to add.
Water pH: The pH of spray water is important because many fungicides and insecticides break down quickly in high pH water. The pH is a measure of the acidity or alkalinity of water, which refers to the number of hydrogen (H+) and hydroxyl (OH–) ions in a solution. The scale for measuring pH runs from 0 to 14. The lower the pH, the more acidic the solution; a higher pH indicates that the solution is more alkaline. Water at pH 7 is neutral — meaning that there are equal numbers of hydrogen and hydroxyl ions in the solution. Many areas in the Midwest have alkaline well water (pH 8.0 or above). The pH of natural surface water sources (ponds, streams, etc.) can vary throughout the season.
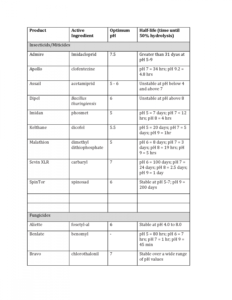
The pH of water can negatively affect the stability of some pesticides. Under alkaline conditions, alkaline hydrolysis occurs, which degrades the pesticide to non-toxic (inactive) forms. In general, insecticides (particularly organophosphates and carbamates) are more susceptible to alkaline hydrolysis than are fungicides, herbicides or growth regulators. The end result is less active ingredient applied and poor pesticide performance. The degradation of a pesticide can be measured in terms of its half-life. For example, if a product has a half-life of 1 hour, its effectiveness is reduced to 50% in 1 hour, to 25% in the next hour, to 12.5% in the next hour, etc. Eventually, the pesticide becomes virtually ineffective. For instance, Captan has a half-life of 32 hours at its optimum pH of 5, but only 10 minutes at pH 8. Similarly, mancozeb has a half-life of 20 days at pH 5, but less than 17 hours at pH 7. Rally (tebuconazole), on the other hand, is not affected by pH.
The effect of pH on pesticides varies from product to product and is also moderated by buffering solutions contained in the pesticide formulation and any adjuvants added to the tank. Tank mixing multiple pesticides can modify the pH of the tank mix.
In general, pesticides are most stable when the spray solution has a pH of about 5. Many water sources are more alkaline than this, so it may be necessary to adjust the pH of the spray solution. There are important exceptions to the rule that spray solutions should be acidified. For instance, in the case of copper-based fungicides, copper becomes more soluble at a lower pH and may become phytotoxic to crops. In addition, phosphorous acid and other acid-based fungicides already have a low pH, and lowering it even more can cause them to injure crops. On the other hand, acidifying carbonate salt fungicides, such as Armicarb, may render them ineffective.
The Midwest Fruit Pest Management Guide has a discussion of spray tank pH. Spray water can be acidified by adding a specific acidifant, or with food grade citric acid. About 2 ounces of food grade citric acid per 100 gallons of water will lower the pH from about 8.0 to about 5.5.
It is important to understand that water pH and hardness are separate issues. Adjusting one may not affect the other. However, several spray adjuvants are available that will affect both hardiness and pH. Discuss your needs with your supplier to be sure you are using the correct products. Read pesticide labels completely and to know the quality of the water used in the sprayer. Test kits for both pH and hardness can be purchased at many box stores. Look in the swimming pool supply isle.